
Lasers, Superconducting Magnets, and More: A Selection of Chemical Instrumentation
Photos by Elena Creed
One of the best aspects of Hillsdale College’s chemistry program is the unparalleled access that students and faculty have to well-equipped lab facilities. Chemistry and biochemistry students regularly get the opportunity to use a wide range of chemical instrumentation. We’ve collected some of the most interesting instruments below – check them out!
* * *
Gas Chromatograph-Mass Spectrometer (GC-MS)

This instrument is used to separate and identify compounds in a mixture. It works by vaporizing a small portion of the sample and passing it through a long capillary column where the separation takes place, largely based on differences in boiling points of the various compounds. Each separated compound is then passed into a mass spectrometer, which ionizes and fragments the compound into pieces that are identifiable by their molecular masses. The pattern and masses of the fragments enables one to identify the compound.
Liquid Chromatograph-Mass Spectrometer (LC-MS)

This instrument is very similar to the GC-MS, except for the fact that the sample is injected as a liquid into a flowing liquid stream, as opposed to being vaporized. It passes through a column where the separation takes place, based primarily on differences in polarity of the various compounds. The mass spectrometer works analogously to the way described for the GC-MS.
Vacuum Atmosphere Glovebox

The glovebox is a hermetically sealed, stainless steel enclosure with a full-view window and 2 glove ports. It provides a leak-tight environment to work with materials that are sensitive to air, moisture, or other common contaminants.
Laser Lab, Surface-Enhanced Raman Spectroscopy (SERS)
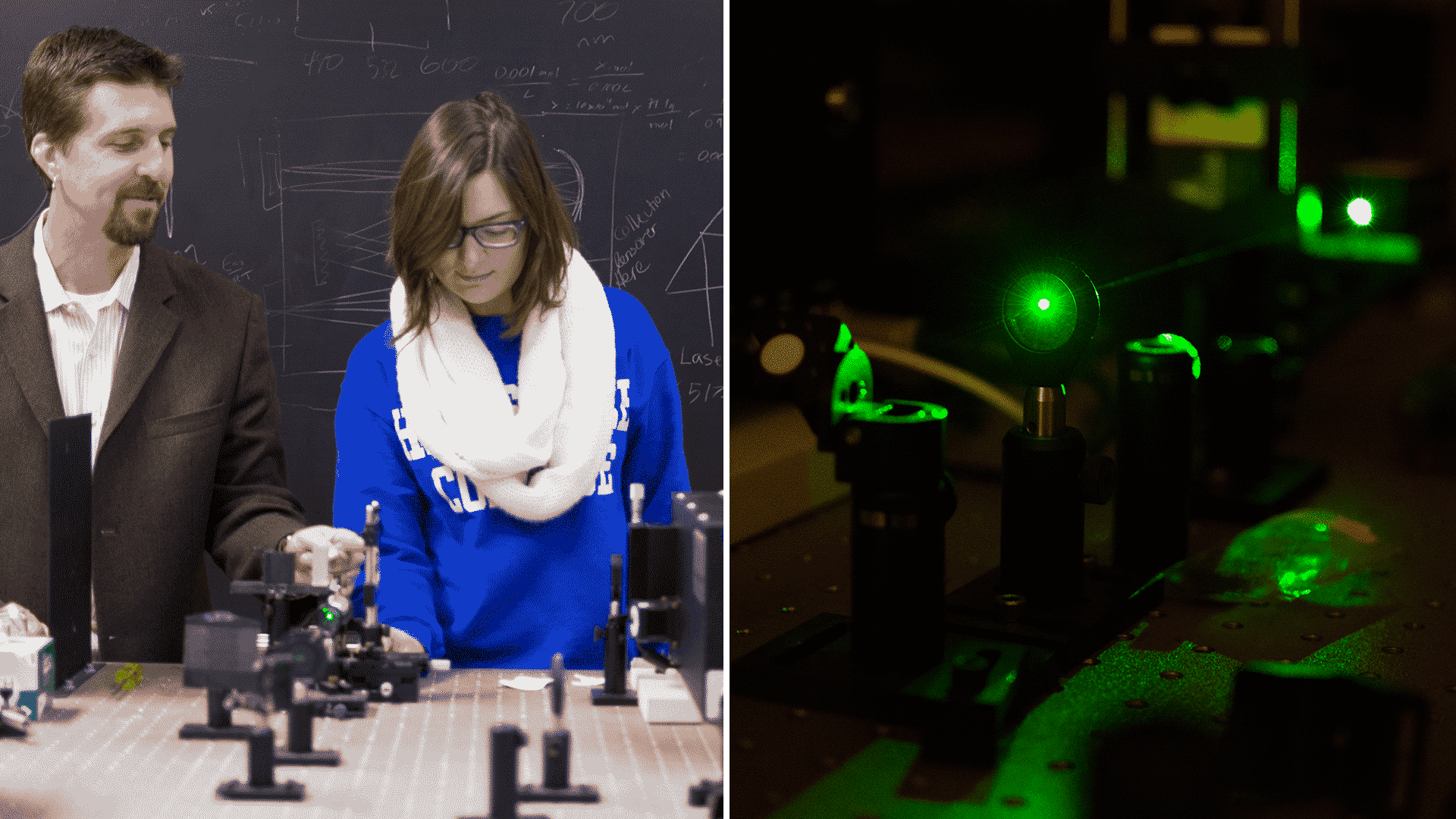
The green laser light is focused on a sample on a surface, and the light that is scattered off of that surface is observed. The surface is modified with nanoparticles, typically silver, that greatly enhance the scattering of light at a wavelength slightly longer than the incident laser beam. The difference in wavelengths between the scattered light and the incident laser provides information related to the molecular structure of the compound on the surface. The intensity of the scattered light at a given wavelength relates to the concentration of the compound.
Nuclear Magnetic Resonance (NMR)

This instrument is similar to an MRI in a hospital, except that it is used to examine the structures of compounds in solution rather than a human body. A small tube containing the sample is placed in an intense superconducting magnetic field, contained in a large tank that uses liquid helium and liquid nitrogen to keep the superconducting magnet very cold. The sample is then irradiated with a broad radio frequency pulse, and the different atomic nuclei in the sample will absorb and re-emit that radio wave at slightly varying frequencies. The differences in frequencies provide structural information on the environment around each nucleus, which enables one to understand the arrangement of chemical bonds and to identify the compound.
Flame Atomic Absorption and Emission
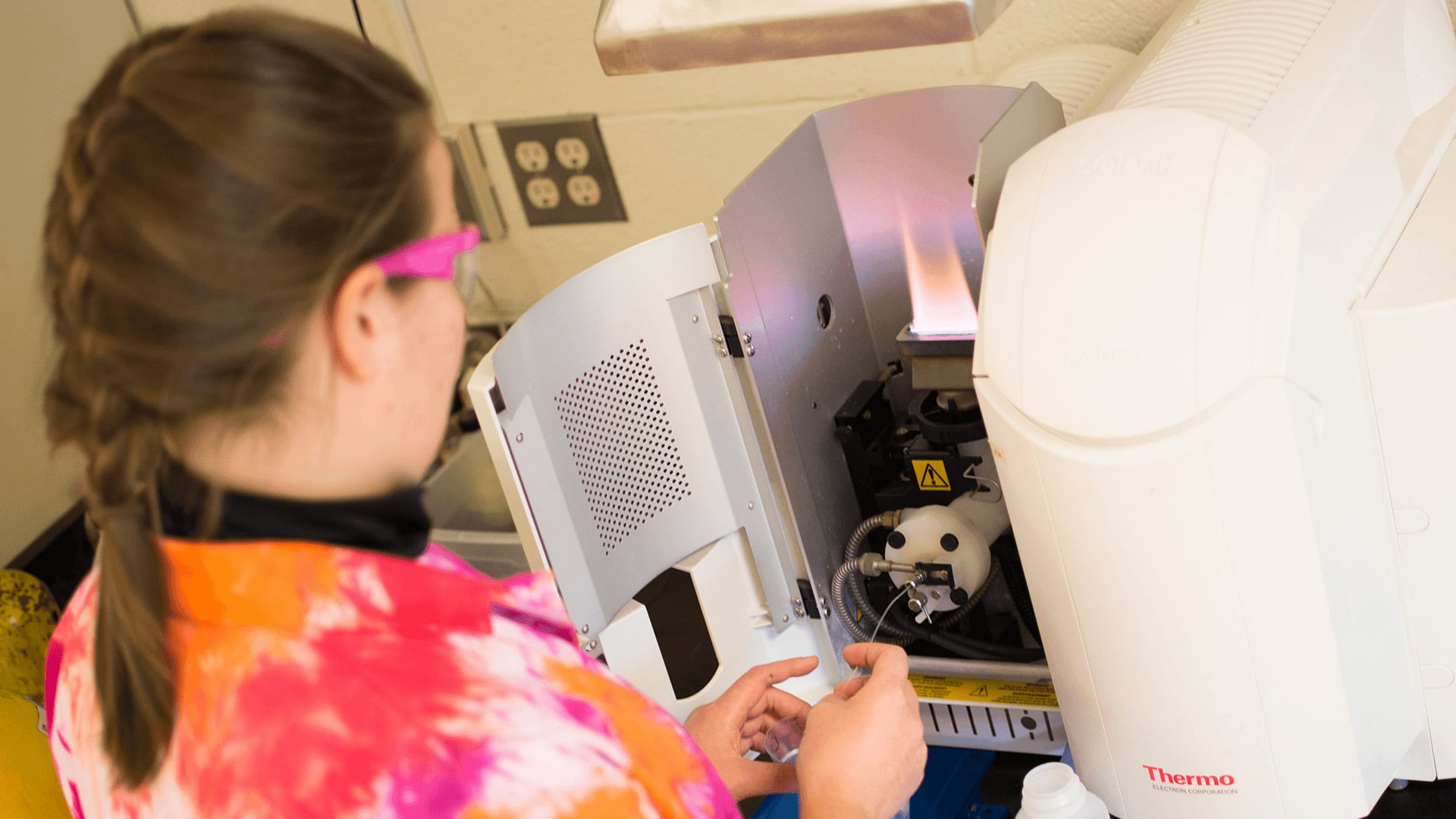
This instrument uses an air-acetylene flame to “excite” atoms in compounds so that they will absorb and/or emit light at specific wavelengths (colors) that are characteristic of the element. By aspirating solutions into the flame, one can use the information on the wavelengths of light absorbed or emitted to identify what elements are present.
Electrochemical Analyzer

This instrument applies a range of voltages to an electrode in chemical solutions to measure how easily electrons are transferred to, or from, different compounds. It can also be used to measure the amount of a particular substance in a given solution – low concentrations of heavy metals in lake water, for example.
* * *
This is just a small sample of all the amazing equipment in the chemistry department. As fascinating as all these instruments are, keep in mind that these tools are only as good as the people operating them. Luckily, we have some of the best, brightest, and fun-loving students and professors in the chemistry department.

 Elena Creed, ’18, plans on double majoring in English and theatre. Hailing from Westford, MA, she loves autumn, colonial history, Dunkin Donuts, Boston, trains, and laughter.
Elena Creed, ’18, plans on double majoring in English and theatre. Hailing from Westford, MA, she loves autumn, colonial history, Dunkin Donuts, Boston, trains, and laughter.